Data Overview
The Michigan Sickle Cell Data Collection (MiSCDC) Program gathers information about people living with sickle cell disease (SCD) in Michigan. MiSCDC aims to use population-level data to inform actions that can be taken to improve the lives and health care of people living with SCD.
Description of Data Sources
MiSCDC program collects data from multiple sources, including data which are maintained by the Michigan Department of Health and Human Services, the Michigan Health & Hospital Association, and clinics providing care and treatment for SCD.
MiSCDC Data Sources
Combining multiple data sources helps create a more complete view of the health information for people with SCD. This comprehensive approach allows us to understand their health care experiences over time.
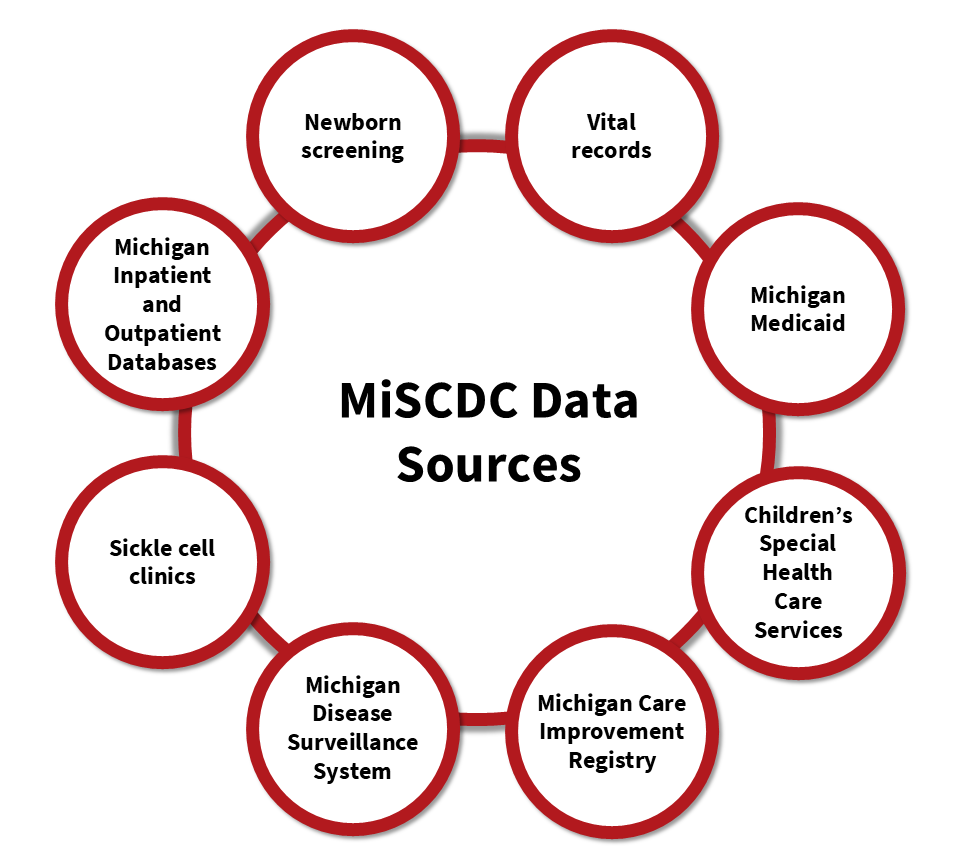
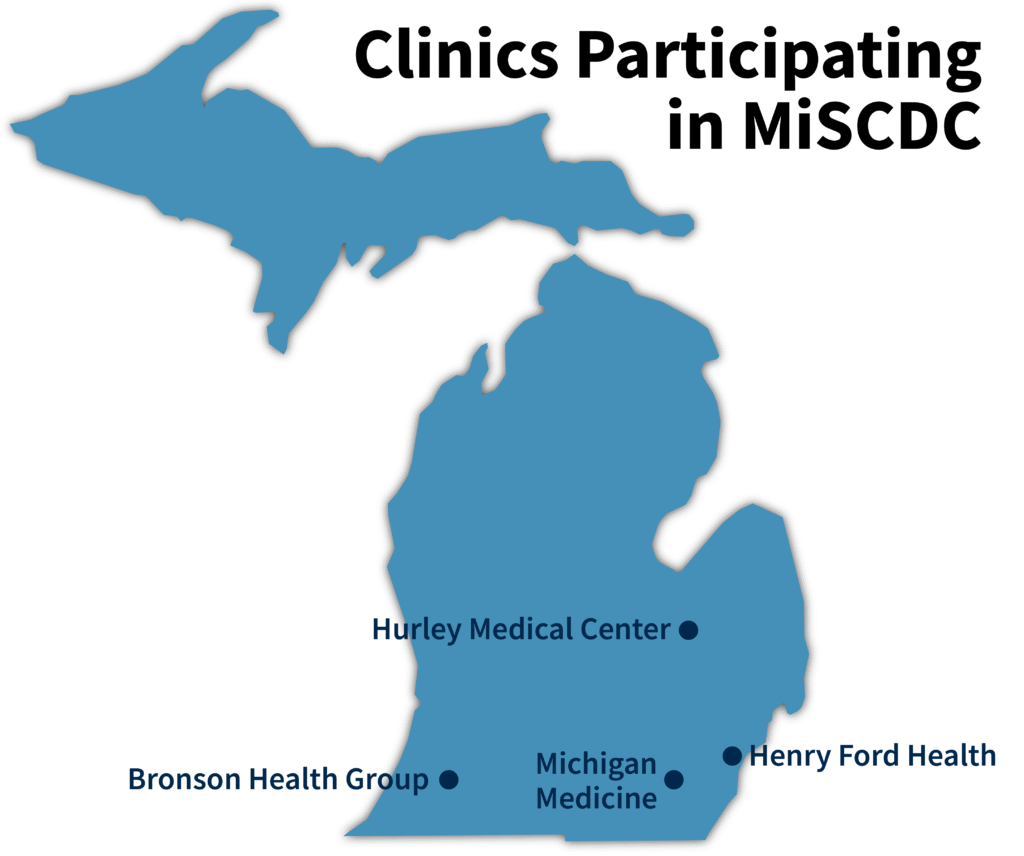
Participating Clinics
MiSCDC obtains demographic and SCD genotype information from these collaborations.
Data Dashboard
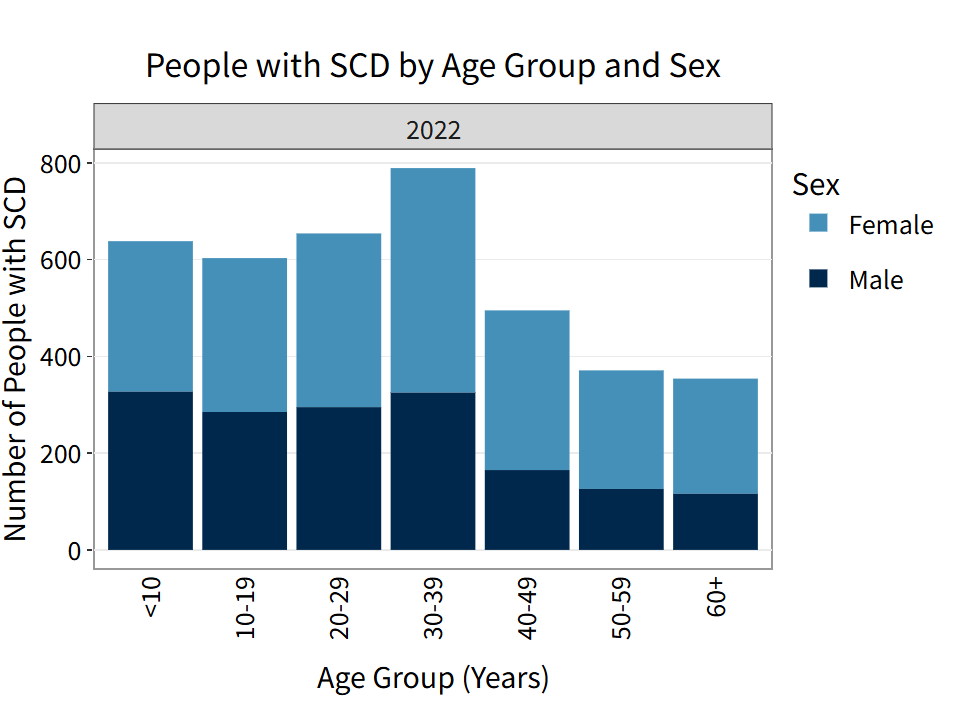
The interactive MiSCDC Data Dashboard includes statistics on births, demographics, geography, and health care utilization of people with SCD in Michigan.
MiSCDC Methods
Click through each tab for an overview of the methods, how individuals living with SCD are identified, and definitions of health measures.
Overview
The following diagram lists the data sources which are combined to form the MiSCDC public health surveillance system for SCD, as well as the outcomes derived from this database. Hover over each box under ‘Data Sources’ for additional information (not available on all browsers).
Defining the Cohort
The goal of MiSCDC is to identify all individuals with SCD living in Michigan. Individuals are identified using the data sources in the overview diagram, and validated SCD case definitions are applied based on the available data (source, source, source – links to external sites).
Newborn Screening records and administrative claims are linked to identify individuals based on genotype. Individuals with a known genotype are placed into the following categories:
- Confirmed SCD: Individual has an SCD genotype in Newborn Screening records or clinic data.
- Does not have SCD: Individual has a non-SCD genotype in Newborn Screening records or clinic data (i.e., sickle cell trait, normal hemoglobin).
For individuals without a genotype recorded in Newborn Screening records, ICD-10 diagnostic codes are used to identify all SCD-related claims (all D57x ICD-10 codes, excluding D57.3). ICD-10 codes are used exclusively from 2017 onward; prior years use different codes from ICD-9. Individuals without a confirmed genotype are categorized into the following:
- Probable SCD Case: Individual has no genotype available but has 3+ SCD visits in a 5-year period across all administrative claims datasets; or has a verified Children’s Special Healthcare Services (CSHCS) diagnosis for SCD.
- Possible SCD Case: Individual has no genotype available but has 1-2 SCD visits in a 5-year period across all administrative claims datasets.
The MiSCDC data dashboard only includes confirmed and probable SCD cases. Each annual cohort consists of individuals who have been classified as a confirmed or probable case and who have evidence of residing in Michigan during that year, or in both the previous and following years.
MiSCDC Measures
The following measures are displayed on the data dashboard. Click on each dropdown to learn how each measure is defined and which data sources are used.
Age and sex of individuals in the cohort are determined through the linkage of Newborn Screening (NBS) records, birth certificates, clinical data, Michigan Medicaid data, and the Michigan Inpatient and Outpatient Databases (MIDB and MODB).
Age is calculated as of December 31st of each year.
Two data sources are utilized to identify inpatient admissions: Michigan Medicaid and Michigan Inpatient Database (MIDB), a hospital discharge database.
In Michigan Medicaid, all claims with an inpatient revenue code are identified. Claims associated with non-acute inpatient care (i.e. skilled nursing facility, psychiatric residential, etc.) are excluded. Inpatient claims are deduplicated by admission and discharge dates; claims with overlapping inpatient dates are combined to retain the earliest admission and latest discharge date. MIDB contains encounter-level inpatient information. This database is used as-is to identify inpatient admissions.
Inpatient admissions for the same individual are deduplicated between data sources by admission and discharge dates; admissions which overlap between sources are combined with the earliest admission and latest discharge dates retained. Inpatient admissions in the MiSCDC data include both direct admissions and admissions that initiated in the emergency department.
Length of stay is the total number of days a patient spends in a healthcare facility from the day of admission through the day of discharge and is calculated for inpatient admissions only. If a patient is admitted and discharged on the same day, the length of stay is counted as one day.
Two data sources are utilized to identify emergency department treat-and-release (ED) visits: Michigan Medicaid and Michigan Outpatient Database (MODB), a hospital discharge database.
In Michigan Medicaid, all claims with current procedural terminology (CPT), healthcare common procedure coding system (HCSPCS), or revenue codes which are ED-related are identified. These claims are deduplicated by admission and discharge dates; claims with overlapping dates are combined to retain the earliest admission and latest discharge date. MODB contains outpatient visits, ED visits, and observation stays at the encounter-level.
ED visits for the same individual are deduplicated between the data sources by admission and discharge dates; visits which overlap between data sources are combined with the earliest admission and latest discharge dates retained. Encounters listed as ED visits in the MiSCDC data only include treat-and-release visits, which are visits that did not lead to an inpatient admission.
To identify 30-day readmissions, combined and deduplicated Michigan Medicaid data and data from the Michigan Inpatient and Outpatient Databases (MIDB and MODB) are utilized.
A 30-day inpatient readmission happens when a person has an inpatient admission within 30 days of being discharged from another inpatient admission. The inpatient readmission rate is the percent of inpatient admissions that have a readmission within 30 days.
A 30-day ED readmission happens when a person has an ED visit within 30 days of being discharged from another ED visit. The ED readmission rate is the percent of ED visits that have another ED visit within 30 days.
To allow for 30 days of follow-up for each encounter, annual 30-day readmission rates only include encounters for which the discharge date is before December 1st of that year.
These definitions differ from the CDC SCDC definition of 30-day readmissions. Always consider the definition of this term if comparing readmission data to SCDC data from other states.
Primary payer is identified from Michigan Medicaid and the hospital discharge databases, Michigan Inpatient Database (MIDB) and Michigan Outpatient Database (MODB). Primary payers are grouped into the following categories: Medicaid, Medicare, Private, and Other.
The Other payer category includes multiple primary payers, unknown or missing payer information, self-pay, auto insurance, worker’s compensation, and Title V.
Frequently Asked Questions
MiSCDC is a state-university partnership between the Michigan Department of Health and Human Services and the University of Michigan Child Health Evaluation and Research (CHEAR) Center. Data collection for MiSCDC is authorized by a grant of public health authority from MDHHS and data use agreements.
In addition, the MiSCDC program and protocol were reviewed by the University of Michigan Medical School Institutional Review Board (HUM00179707) and determined to be Not Regulated, as the project consists of standard public health surveillance activities.
Data security is of utmost importance to MiSCDC. MiSCDC and MDHHS have developed data use agreements, which specify data sources approved for acquisition and all required data security measures. All data are acquired, transferred, and stored through secure, HIPAA-compliant applications.
Data across all data sources are linked together using unique identifiers whenever possible. In the absence of such identifiers, data linkage software is used to probabilistically link datasets based on information that is available, such as demographic characteristics.
Record linkage identifies records belonging to the same person across data sources. Deduplication ensures that the same individual or health care utilization are not counted more than once. Regularly linking and deduplicating data improves the completeness and accuracy of the database.
All aggregate, publicly available data can be viewed and downloaded on our data dashboard. Aggregate data across all SCDC partnering states can also be seen at the CDC website.
Note: Individual-level data will never be available publicly.
Publicly-available aggregate data can be viewed and downloaded on our data dashboard page. This data can be used with proper citation. If the publicly-available data does not fit your needs, please visit our Contact Us page or email us at MichiganSCDC@umich.edu with information about your request.
Please visit our Contact Us page or email us at MichiganSCDC@umich.edu. Never send PHI or medical information via the Contact Us page or email.
Glossary
Population-level data
Data which are collected across a specific population. For example, the total hospital admissions among individuals living with SCD. Population-level data is collected by MiSCDC for the purposes of public health surveillance, which is defined as “activities that allow a public health authority to identify, monitor, assess, or investigate potential public health signals, onsets of disease outbreaks, or conditions of public health importance that are conducted, supported, requested, ordered or required (by) and authorized by a Public Health Agency or authority of the United States 45 C.F.R.§ 46.102(l)(2)” (source- links to external site). Public health surveillance is not research. Activities within a surveillance system are limited to those that are necessary for the identification and monitoring of conditions of public health importance.
Public health authority
A grant of public health authority is an official mandate that allows MiSCDC to act on behalf of MDHHS to conduct SCD public health surveillance within the state (source- links to external site).
Administrative claims datasets
Administrative claims datasets contain information from bills and payments between doctors and insurance companies. They include details about medical services, medications, and diagnostic codes. These datasets can be used in public health surveillance to understand health care outcomes.
ICD-10
The International Classification of Diseases, 10th Revision, is a system of medical codes that physicians use to classify all diagnoses, symptoms, and procedures for insurance claims processing (source- links to external site).
Data use agreement
A contractual agreement that establishes guidelines for securing sensitive data and transferring data among stakeholders (source- links to external site).
